Project Context
A client aimed to integrate two new 800L mobile vessels into its production line for monoclonal antibody-based vaccines. The project required seamless implementation in an active GMP-regulated environment while ensuring equipment standardization and full data integrity.
RealDev's Mission
RealDev was entrusted with leading and supervising the integration at multiple levels:
Infrastructure Preparation
- Supervision of utility connections, including the installation of a customized Harting socket column
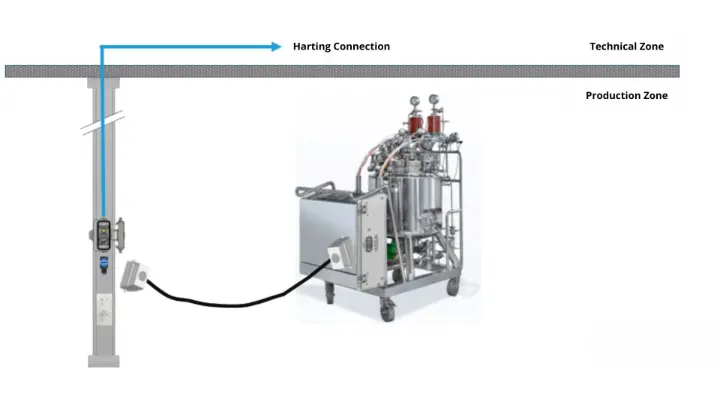
PLC Programming
- Development of PLC logic using Siemens TIA Portal, in accordance with the client’s standardization strategy for all vessels
- Integration of the new vessels into the central control PLC
Supervision and Data Collection
- Implementation of real-time data communication from the new vessels to the central PLC (PLC centralisateur), and then to the IFIX supervisory system
- Setup of data historization to ensure compliance with traceability and regulatory standards
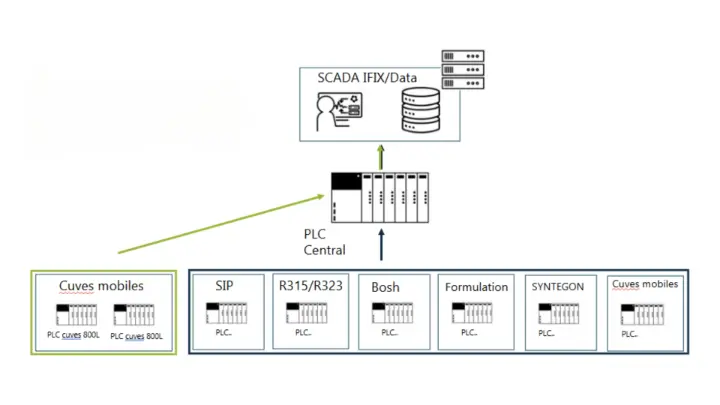
Retrieval of existing data
New data upload
Expected Outcomes
- A validated, compliant process aligned with GMP and data integrity standards
- Seamless equipment integration with minimal impact on production

Looking to optimize your production processes?
Whether you need to migrate existing systems, integrate new equipment, or ensure full compliance and validation, RealDev is your trusted partner in industrial automation and life sciences. Contact us today to discuss how we can support your project!




